Tirzepati(mounja)5mg 10mg 15mg injection
irzepatide
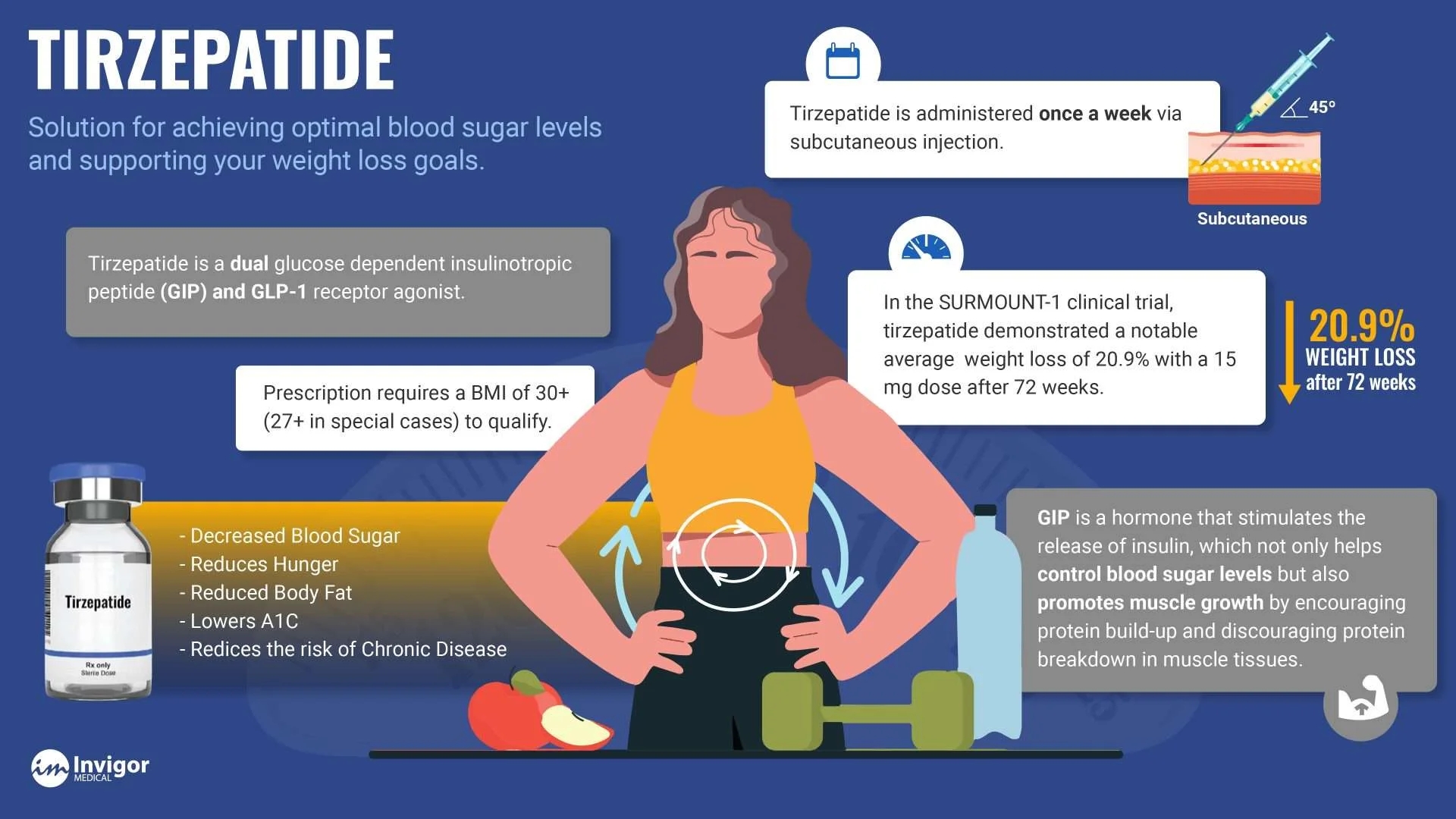
Tirzepide is an investigational once-weekly, dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist that integrates the actions of both incretins into a single molecule.rzepatide Mimics Natural Hormones that Foster Feeling Full
Tirzepatide works by mimicking the GLP-1 and GIP hormones that are naturally secreted by the intestine after a meal, which prompts insulin secretion. It also reduces appetite by slowing down the time it takes the stomach to empty and interacting with areas in the brain harboring GLP-1 receptors to signal satiety.
Tirzepatide is a novel medication that is FDA approved for the treatment of type 2 diabetes mellitus. Given its potent weight loss properties, tirzepatide be used off-label for obesity treatment. It works as a dual GLP-1 agonist and GIP agonist to maximize similar benefits that are seen with GLP-1 medications such as semaglutide. It is currently implemented as a second-line diabetes medication, similar to GLP-1 medications, and given as a once-a-week subcutaneous injectable.
rzepatide is a glucose-dependent insulinotropic polypeptide (GIP) receptor and glucagon-like peptide-1 (GLP-1) recepto agonist, which is FDA-approved for treating type 2 diabetes mellitus. It is important to note that tirzepatide is not approved for treating type-1 diabetes mellitus and has not been studied in patients with pancreatitis. Tirzepatide is a GIP receptor and GLP-1 receptor agonist, leading to significantly improved glycemic control in type 2 diabetics and significant weight reduction.
The FDA approved Tirzepatide in May 2022. Tirzepatide can also be used off-label for treating obesity. It is currently implemented as a second-line diabetes medication, similar to GLP-1 medications like semaglutide. It is a once-a-week subcutaneous injectable medication with incremental dose increases.
Current clinical data has demonstrated that tirzepatide is superior to placebo in improving hemoglobin A1C levels. The SURPASS-5 clinical trial showed a -2.11% reduction in hemoglobin A1C levels at 5mg per week dosing, compared to -0.86% with placebo. At the highest dose of 15 mg per week, tirzepatide led to a -2.34% reduction in hemoglobin A1C. This was demonstrated over 40 weeks. A weight reduction of 5.4 kg was seen with 5mg of tirzepatide dosing, and a 10.5 kg reduction was seen with 15 mg dosing. This dose-dependent correlation with weight loss is similar to semaglutide, a common GLP-1 medication utilized for weight loss management.
Comparatively, tirzepatide has been shown to work similarly to GLP-1 medications but with greater efficacy. Given its weight loss properties and lack of liver toxicity, it is likely to play an indirect role in the treatment of nonalcoholic fatty liver disease (NAFLD) as well.
NOTE
We ship all over the world.
You are advised to consult your medical consultations before use of the product.