Article introduction:
NAD+ is essential to the creation of energy in the body and the regulation of pivotal cellular processes. Here’s why it’s so important, how it was discovered, and how you can get more of it.
How NAD+ Is Powerful
Open any biology textbook and you’ll learn about NAD+, which stands for nicotinamide adenine dinucleotide. It’s a critical coenzyme found in every cell in your body that’s involved in hundreds of metabolic processes like cellular energy and mitochondrial health. NAD+ is hard at work in the cells of humans and other mammals, yeast and bacteria, even plants.
Scientists have known about NAD+ since it was first discovered in 1906, and since then our understanding of its importance has continued to evolve. For example, the NAD+ precursor niacin played a role in mitigating pellagra, a fatal disease that plagued the American south in the 1900s. Scientists at the time identified that milk and yeast, which both contain NAD+ precursors, alleviated symptoms. Over time scientists have identified several NAD+ precursors — including nicotinic acid, nicotinamide, and nicotinamide riboside, among others — which make use of natural pathways that lead to NAD+. Think of NAD+ precursors as different routes you can take to get to a destination. All the pathways get you to the same place but by different modes of transportation.
Recently, NAD+ has become a prized molecule in scientific research because of its central role in biological functions. The scientific community has been researching how NAD+ relates to notable benefits in animals that continue to inspire researchers to translate these findings to humans. So how exactly does NAD+ play such an important role? In short, it’s a coenzyme or “helper” molecule, binding to other enzymes to help cause reactions on the molecular level.
But the body doesn’t have an endless supply of NAD+. In fact, it actually declines with age. The history of NAD+ research, and its recent establishment in the science community, has opened the floodgates for scientists to investigate maintaining NAD+ levels and getting more NAD+.
What is the History of NAD+?
NAD+ was first identified Sir Arthur Harden and William John Young in 1906 when the two aimed to better understand fermentation — in which yeast metabolize sugar and create alcohol and CO2. It took nearly 20 years for more NAD+ recognition, when Harden shared the 1929 Nobel Prize in Chemistry with Hans von Euler-Chelpin for their work on fermentation. Euler-Chelpin identified that the structure of NAD+ is made up of two nucleotides, the building blocks for nucleic acids, which make up DNA. The finding that fermentation, a metabolic process, relied on NAD+ foreshadowed what we now know about NAD+ playing a critical role in metabolic processes in humans.
Euler-Chelpin, in his 1930 Nobel Prize speech, referred to NAD+ as cozymase, what it was once called, touting its vitality. “The reason for our doing so much work on the purification and determination of the constitution of this substance,” he said, “is that cozymase is one of the most widespread and biologically most important activators within the plant and animal world.”
Otto Heinrich Warburg — known for “the Warburg effect” — pushed the science forward in the 1930s, with research further explaining NAD+ playing a role in metabolic reactions. In 1931, the chemists Conrad A. Elvehjem and C.K. Koehn identified that nicotinic acid, a precursor to NAD+, was the mitigating factor in pellagra. United States Public Health Service Doctor Joseph Goldberger had previously identified that the fatal disease was connected to something missing in the diet, which he then called PPF for “pellagra preventive factor.” Goldberger died before the ultimate discovery that it was nicotinic acid, but his contributions led to the discovery, which also informed eventual legislation mandating the fortification of flours and rice on an international scale.
The next decade, Arthur Kornberg, who later won the Nobel Prize for showing how DNA and RNA are formed, discovered NAD synthetase, the enzyme that makes NAD+. This research marked the beginning of understanding the building blocks of NAD+. In 1958, the scientists Jack Preiss and Philip Handler defined what’s now known as the Preiss-Handler pathway. The pathway shows how nicotinic acid — the same form of vitamin B3 that helped cure pellagra — becomes NAD+. This helped scientists further understand the role of NAD+ in the diet. Handler later earned the National Medal of Science from President Ronald Reagan, who cited Handler’s “outstanding contributions to biomedical research…furthering the state of American science.”
While scientists had now realized the importance of NAD+, they had yet to discover its intricate impact on a cellular level. Forthcoming technologies in scientific research combined with comprehensive recognition of the coenzyme’s importance ultimately encouraged scientists to continue studying the molecule.
How does NAD+ work in the body?
NAD+ works as a shuttle bus, transferring electrons from one molecule to another within cells to carry out all sorts of reactions and processes. With its molecular counterpart, NADH, this vital molecule participates in various metabolic reactions that generate our cell’s energy. Without sufficient NAD+ levels, our cells wouldn’t be able to generate any energy to survive and carry out their functions. Other functions of NAD+ include regulating our circadian rhythm, which controls our body’s sleep/wake cycle.
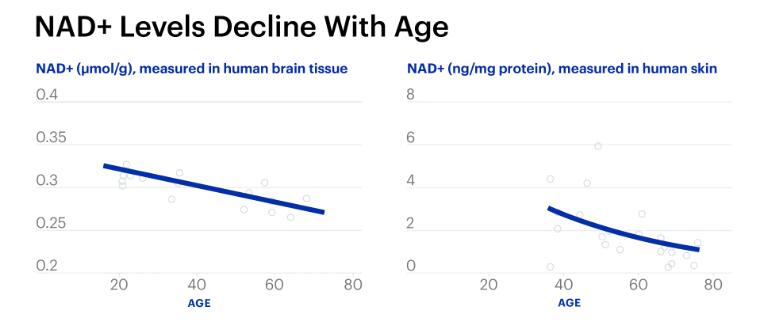
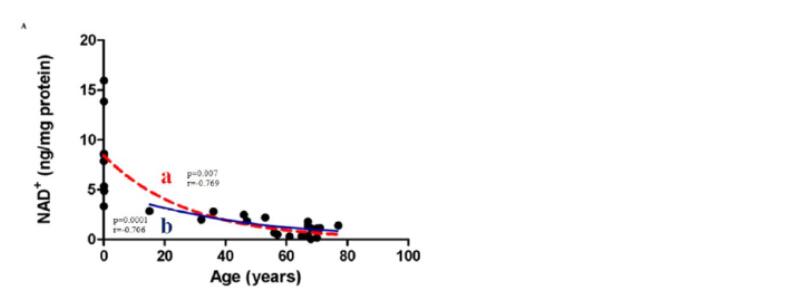
As we age, NAD+ levels fall, suggesting important implications in metabolic function and age-related diseases. DNA damage accumulates and snowballs with aging.
What happens when NAD+ levels are reduced?
Numerous studies demonstrate reduced NAD+ levels in disturbed nutrient conditions, such as obesity, and aging. Reductions in NAD+ levels can lead to problems with metabolism. These problems can lead to disorders, including obesity and insulin resistance. Obesity causes diabetes and high blood pressure.
Metabolic disorders caused by the low NAD+ level cascade down. High blood pressure and other heart function decline can send damaging pressure waves to the brain that may lead to cognitive impairment.
Targeting NAD+ metabolism is a practical nutritional intervention in protecting against metabolic and other age-related diseases. Several groups have done studies indicating supplementing with NAD+ boosters improves insulin resistance from obesity. In mouse models of age-related diseases, supplementing with NAD+ boosters improves symptoms of the diseases. This suggests reduced NAD+ levels with age may contribute to the onset of age-related diseases.
Preventing the decline of NAD+ offers a promising strategy to combat metabolism disorders with age. As NAD+ levels decrease with age, this can lead to reduced DNA repair, cellular stress response, and regulation of energy metabolism.
Potential Benefits
NAD+ is important for species’ mitochondrial maintenance and gene regulation regarding aging. However, the level of NAD+ in our body declines drastically with age. “As we get older, we lose NAD+. By the time you’re 50, you have about half the level you once had when you were 20,” says David Sinclair of Harvard University in an interview.
Studies have shown the decrease of the molecule associates with age-related diseases including accelerated aging, metabolic disorders, heart disease, and neurodegeneration. Low levels of NAD+ is associated with age-related disease due to less functional metabolism. But replenishing NAD+ levels has presented anti-aging effects in animal models, showing promising results in reversing age-related diseases, increasing lifespan and healthspan.
Aging
Known as the “guardians of genomes,” sirtuins are genes that protect organisms, from plants to mammals, against deterioration and diseases. When the genes sense the body is under physical stress, such as exercising or hunger, it sends out troops to defend the body. Sirtuins sustain genome integrity, promote DNA repair and have shown anti-aging related properties in model animals like increasing lifespan.
NAD+ is the fuel that drives the genes to work. But like a car cannot drive without its fuel, sirtuins require NAD+. Results from studies show that raising NAD+ level in the body activates sirtuins and increases lifespan in yeast, worms, and mice. Although NAD+ replenishing shows promising results in animal models, scientists are still studying how these results can translate to humans.
Muscle function
As the powerhouse of the body, mitochondrial function is crucial for our exercise performance. NAD+ is one of the keys to maintaining healthy mitochondria and steady energy output.
Increasing NAD+ levels in muscle can improve its mitochondria and fitness in mice. Other studies also show that mice that take NAD+ boosters are leaner and can run farther on the treadmill, showing a higher exercise capacity. Aged animals that have a higher level of NAD+ outperforms its peers.
Metabolic disorders
Declared as an epidemic by the World Health Organization (WHO), obesity is one of the most common diseases in modern society. Obesity can lead to other metabolic disorders such as diabetes, which killed 1.6 million people around the globe in 2016.
Aging and high-fat diet reduce the level of NAD+ in the body. Studies have shown that taking NAD+ boosters can alleviate diet-associated and age-associated weight gain in mice and improve their exercise capacity, even in aged mice. Other studies even reversed the diabetes effect in female mice, showing new strategies to fight metabolic disorders.
Heart function
The elasticity of the arteries acts as a buffer between pressure waves sent out by heartbeats. But arteries stiffen as we age, contributing to high blood pressure, the most important risk factors for cardiovascular disease. One person dies from cardiovascular disease every 37 seconds in the United States alone, CDC reports.
High blood pressure can cause an enlarged heart and blocked arteries that lead to strokes. Boosting NAD+ levels gives protection to the heart, improving cardiac functions. In mice, NAD+ boosters have replenished NAD+ levels in the heart to baseline levels and prevented injuries to the heart caused by a lack of blood flow. Other studies have shown that NAD+ boosters can protect mice from abnormal heart enlargement.
Does NAD+ increase lifespan?
Yes, it does. If you were a mouse. Increasing NAD+ with boosters, such as NMN and NR, can extend lifespan and healthspan in mice.
Increased NAD+ levels give a modest effect with extending lifespan in mice. Using the NAD+ precursor, NR, scientists find in a study published in Science, 2016, NR supplementation increases mice’s lifespan by roughly five percent.
Boosted NAD+ levels also confer protection against various age-related diseases. Protection against age-related diseases means living a healthier life for a longer period, increasing healthspan.
In fact, some anti-aging scientists like Sinclair consider the results in the animal study successful that they, themselves, are taking NAD+ boosters. However, other scientists like Felipe Sierra of the national institute on aging at NIH don’t think the drug is ready. “The bottom line is I don’t try any of these things. Why don’t I? Because I’m not a mouse,” he said.
To mice, the search of the “fountain of youth” might have come to an end. However, for humans, scientists agree that we are not quite there yet. Clinical trials of NMN and NR in humans may provide results in the next few years.
The Future of NAD+
As the “silver wave” rolls in, a solution for age-associated chronic diseases to lift the health and economic burden becomes urgent. Scientists may have found a possible solution: NAD+.
Dubbed the “miracle molecule” for the capability to restore and maintain cellular health, NAD+ has shown various potentials in treating heart diseases, diabetes, Alzheimer’s, and obesity in animal models. However, understanding how studies in animals can translate to humans is the next step for scientists to ensure the safety and efficacy of the molecule.
Scientists aim to fully understand the biochemical mechanism of the molecule and the research on NAD+ metabolism continues. The details of the molecule’s mechanism may unveil the secret to bringing anti-aging science from bench to bedside.
Post time: May-17-2024